Business
Stem Cell Therapy
Organoid
Stem Cell and CDMO Business
R&D
Core Technology
FURESTEM-OA Kit Inj., a therapeutic biological product, is composed of allogeneic umbilical cord blood-derived mesenchymal stem cells (FURESTEM-OA Inj.), as a major active component for repairing tissue damage, and cartilage acellular matrix (CAM Inj.), a medical device, as a supportive material.


OSCA is an advanced bio-convergence therapeutic product for the treatment of osteoarthritis, composed of human umbilical cord blood–derived mesenchymal stem cells and porcine cartilage–derived acellular cartilage matrix.
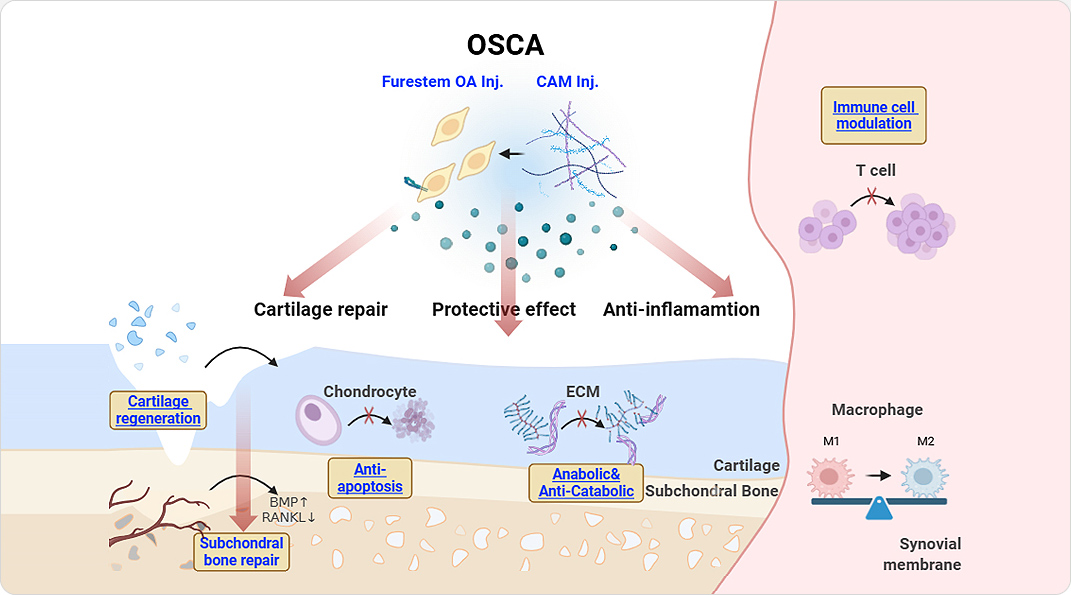
Following intra-articular administration, CAM Inj., enhances the secretion of active factors from the stem cell, thereby triggering cartilage and subchondral bone regeneration, protection of intra-articular tissues, and anti-inflammatory effects.
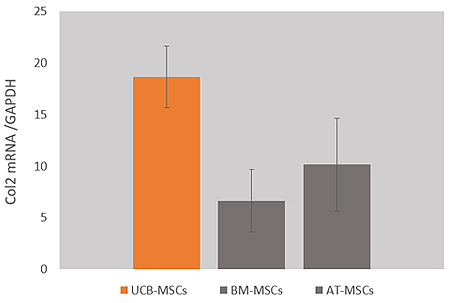
Chondrogenic
differentiation potential
NOD2
expression levels
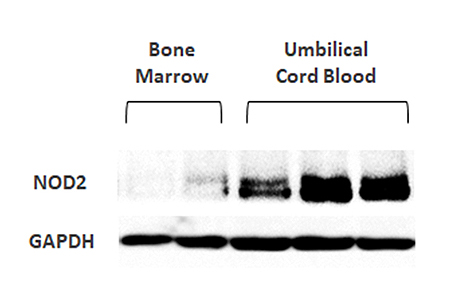
FURESTEM-OA Inj. is a stem cell product that has been confirmed to exhibit superior chondrogenic differentiation potential and higher expression levels of immunomodulatory factors compared with stem cells derived from other tissue sources.
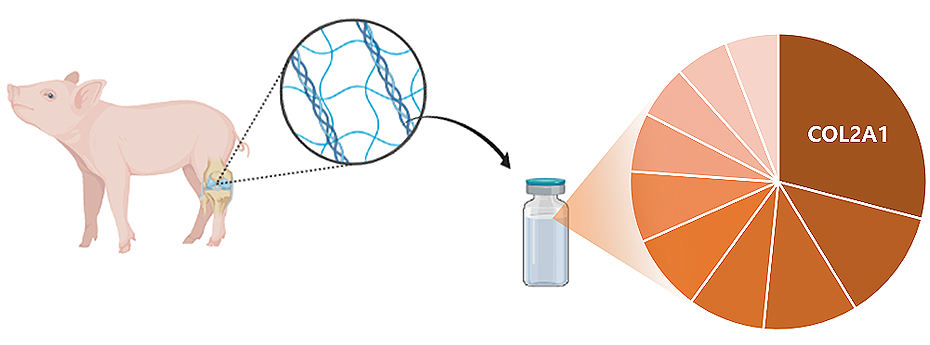
CAM Inj. uses porcine cartilage–derived acellular extracellular matrix as its primary material and is composed of components highly similar to those of native cartilage matrix.
![Amplification of stem cell efficacy by CAM Inj.]](/imgs/business/en/furestemOA05.png)
Stem cells treated with CAM Inj. exhibit increased TGF-β1 secretion, thereby enhancing signaling pathways that promote chondrocyte differentiation. In addition, a microenvironment conducive to cartilage tissue formation is established.

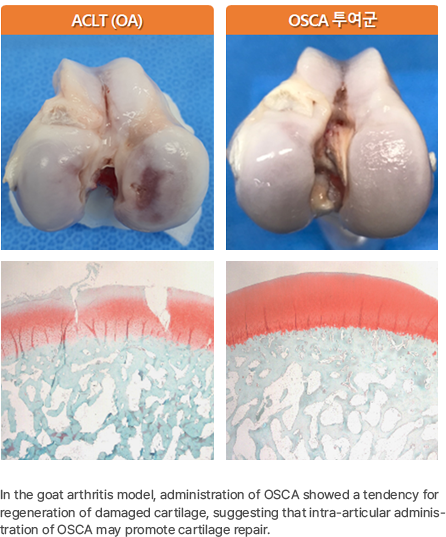
In a goat model of osteoarthritis, administration of OSCA resulted in a regenerative trend in damaged cartilage, suggesting that intra-articularly administered OSCA has the potential to promote cartilage repair.
ACLT (OA)
+ OSCA
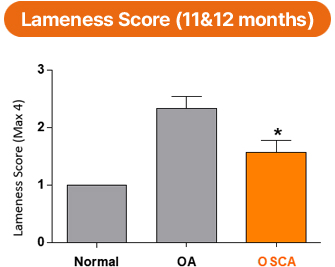
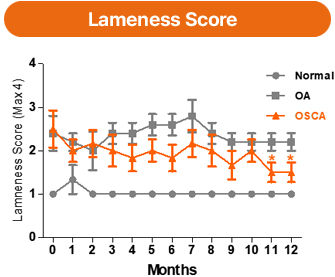
In gait (lameness) assessments in a goat osteoarthritis model, the OSCA-treated group showed a more favorable trend in joint function recovery, which was maintained for up to 12 months.
Stem Cell Therapy
Organoid
Stem Cell and CDMO Business
Core Technology