Business
Stem Cell Therapy
Organoid
Stem Cell and CDMO Business
R&D
Core Technology
Kanstem Biotech provides CDMO services, including contract development, contract manufacturing, and technical consulting, based on over 10 years of accumulated experience in cell therapy development, extensive operational know-how, highly skilled professionals, and its proprietary SELAF platform

Extensive Manufacturing Technologies and Expertise
State-of-the-Art GMP Facilities Compliant with Global Standards
Contract development
Contract Manufacturing
Clinical Research for Advanced Regenerative Medicine
Contract Development for Filling and Cryopreservation
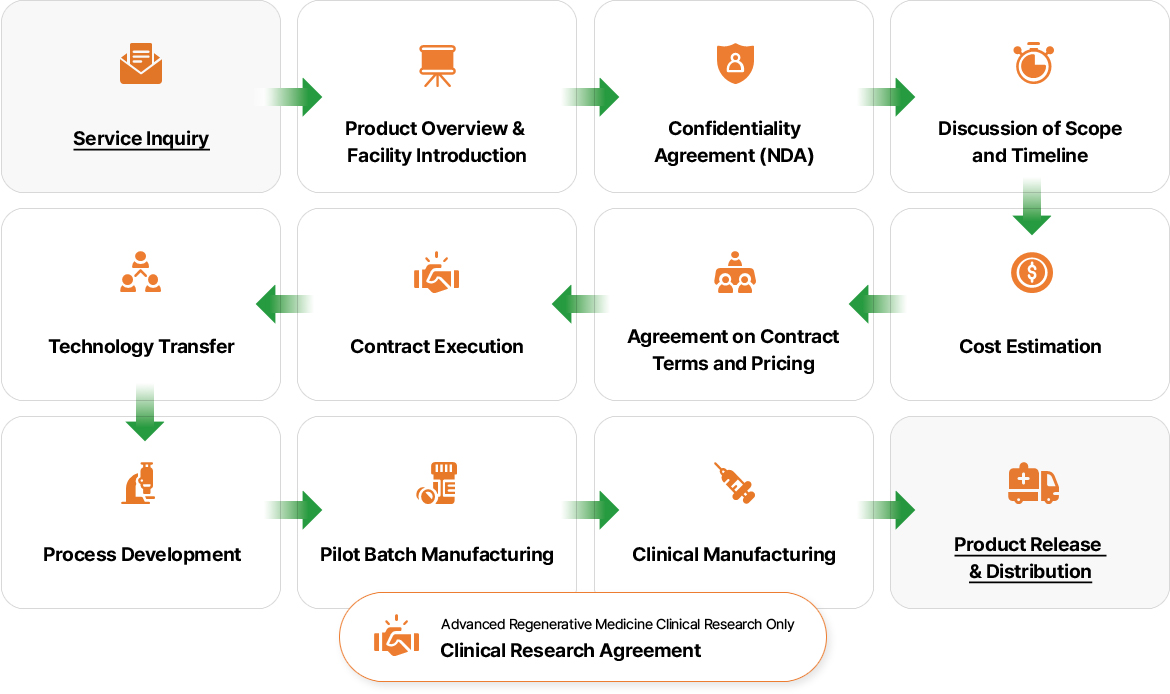

For CDMO-related inquiries and requests, please click the “Service Inquiry” button on the right, complete the inquiry form, and send it to us via email at sales@kangstem.com. Our team will review your request and respond with detailed information.
# To ensure prompt handling, please include “[CDMO]” at the beginning of your email subject line.
(e.g., [CDMO] Inquiry regarding CDMO services)
Stem Cell Therapy
Organoid
Stem Cell and CDMO Business
Core Technology